

Table 4.3 N-term results for the Cn dipole dispersion constant and the Cg London dispersion coefficients for the H-H interaction. The coupling between the different components of the polarizabilities occurs through the denominator in the London formula (4.19), so that we cannot sum over i or / to get the full, observable,12 aA oraB. Polarisability of interacting particlesĪns.In this way, for each H atom, the calculated dipole pseudospectra a, si] i = 1,2,, N of Table 4.2 can be used to obtain better and better values for the C6 London dispersion coefficient for the H-H interaction a molecular (two-centre) quantity C6 can be evaluated in terms of atomic (one-centre), nonobservable, quantities, a, (a alone is useless).
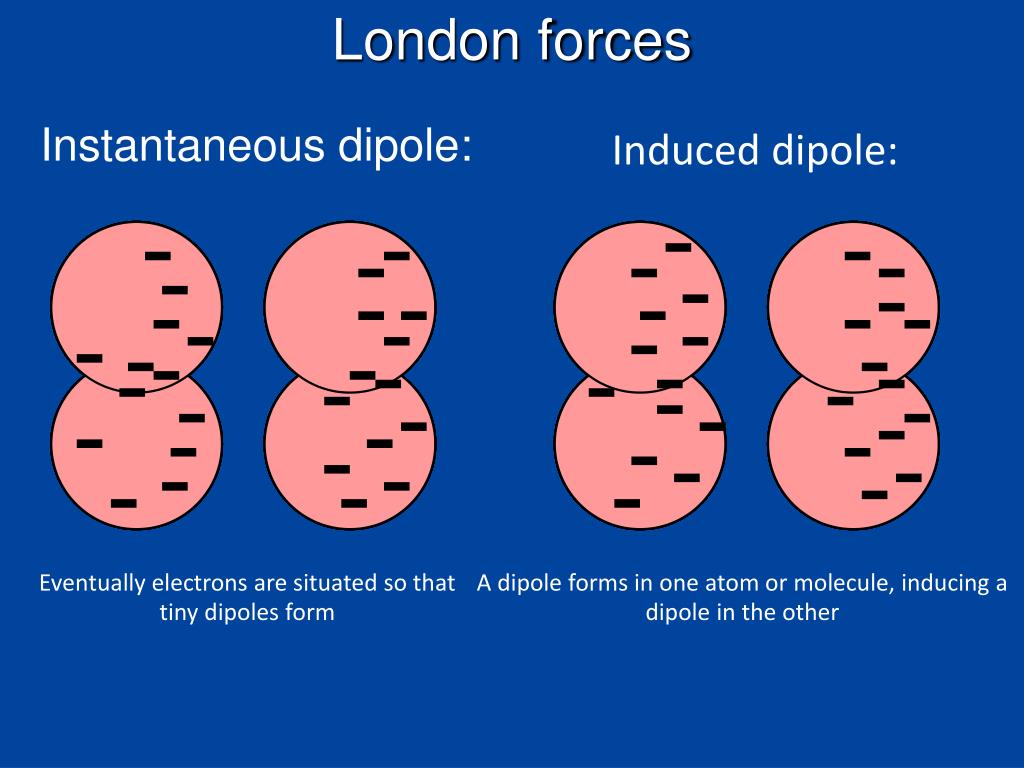
strength of permanent dipoles in the particles.The interaction energy of London force is inversely proportional to the sixth power of the distance between two interacting particles but their magnitude depends upon: The London dispersion force acts as a major intermolecular force of attraction in which of the following? dispersion force is the only intermolecular force that works on what?Īns. What type of intermolecular forces are due to the attraction between temporary dipoles and their induced temporary dipoles? What is the pulse dispersion per unit length, if for a graded-index fiber, 0.1μs pulse broadening is seen over a distance of 13 km? C) Overlapping of light pulses on compression Overlapping of light pulses on compressionĪns.Broadening of transmitted light pulses along the channel.What is dispersion in optical fiber communication? Examples of those types of alkenes are shown in the table below. The alkene isomers whose branches are less developed have more boiling points and whose branches are more developed having more less boiling points. And that’s the reason for decreasing the boiling point of the molecules. R is the distance between the molecules Dispersion forces: ExamplesĪs we know dispersion forces are framed on the small surface area. The interaction energy can be calculated using the London dispersion force formula as follow:Įquation 1 was designed for 2 identical atoms/ molecules which was further modified by a German Physicist for 2 un identical atoms/molecules as follows: It is represented by ‘μ’ and the expression for the same is: The tendency of the molecules to form induced dipoles is known as Polarizability. Dispersion forces may be attractive or repulsive types of forces.Dispersion forces are present in between the long-range molecules greater than nanometer distances.The main principle behind the dispersion forces is the order of the magnitude of attractive forces.The reason behind it is that I atoms have more electrons than the Cl atoms and that’s the reason behind the greater dispersion forces.

For example, we can say that HCl has a lower boiling point than HI but HCl has a more dipole moment than the HI.Dispersion forces are always present whether the dipole moment is present or not.The stronger the dispersion force in between solute molecules and solvent molecules, the greater solubility in between the solvent and solute. So, we can say that more energy is required to separate the two molecules which are connected by dispersion forces. So, the stronger dispersion forces create more energy. Remember that, “ Dispersion forces are inversely proportional to the sixth power of the distance between interacting atoms or molecules”.ĭispersion forces are framed in liquid states.The reason behind that is they are connected because of electronegativity.



 0 kommentar(er)
0 kommentar(er)
